
One preliminary study suggests CBD may help to fight Covid-19
Further studies will be required to verify the research and facilitate the creation of CBD-centric solutions suitable for Covid-19 patients.

Further studies will be required to verify the research and facilitate the creation of CBD-centric solutions suitable for Covid-19 patients.

The European Commission approved GW Pharma's Epidyolex as a treatment for seizures in two rare and severe forms of epilepsy. The drug won FDA approval last year.

How Apella leverages technology to increase OR efficiency.

The Canadian company's shares reached a high price after it signed a framework agreement with Novartis's generics arm that would enable global commercialization.
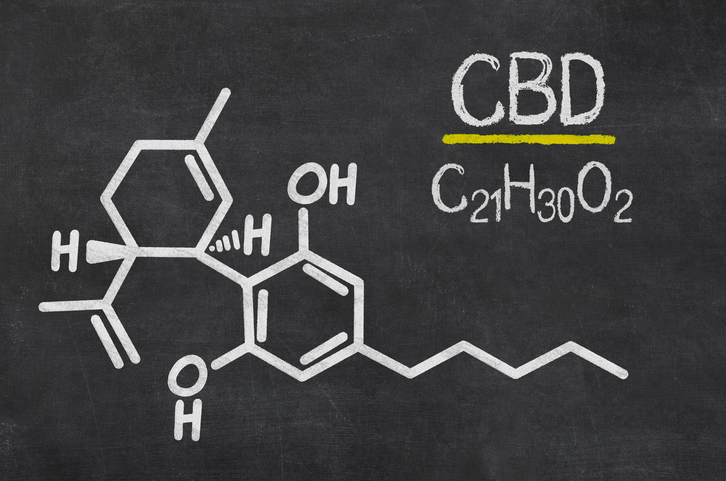
The FDA approved the cannabis-based drug for treating seizures in two severe forms of epilepsy.

From eye drops for glaucoma to chewing gum for irritable bowel syndrome, derivatives of cannabis are making their way into FDA trials in pursuit of a biotech/pharma market projected to reach $20 billion by 2020.